Table of contents

When a perfect CoA still gives a bad powder day
A truck arrives, the paperwork looks flawless, and the powder certificate of analysis (CoA) ticks every required box. Operations relax, and the material goes into silos, hoppers, or feeders without much discussion about risk. On paper, the batch meets specification, yet in the plant, it sometimes behaves like a completely different product.
Problems rarely appear at the gate, because discharge often looks normal during the first moments after intake. They emerge as slow flow, erratic feeding, unexpected segregation, or caking during storage, sometimes weeks after delivery. Teams adjust setpoints, blame the dryer or the mixer, and occasionally question the silo design and venting quality. In many cases, the real issue is simpler, because the plant trusted the document more than the actual powder.
For simple liquids and well-defined chemicals, a certificate of analysis can describe behavior reasonably well in daily operation. For powders, that assumption is risky because behavior depends on structure, handling history, and environment from intake through processing. These factors rarely appear in the certificate and seldom capture plant-specific realities that drive true performance.
What is a powder certificate of analysis in practice?
In practice, a powder certificate of analysis is a structured document that confirms identity, composition, and a limited set of physical properties for an incoming batch. It usually reports results from standardised methods agreed between supplier and customer within long-standing quality agreements. The certificate helps to prove that the batch meets predefined limits, but it does not describe every aspect of powder behavior that matters during operation.
Most supplier certificates follow a familiar pattern developed originally for general chemicals. They confirm identity, list assay, report one particle size distribution, and include moisture content measured under a single specified condition. Some add bulk density, pH, or microbiology where relevant for safety or regulatory reasons in the receiving plant. These parameters confirm chemistry and basic safety, yet they say very little about how the powder will flow, fluidise, compact, or segregate in your process equipment.
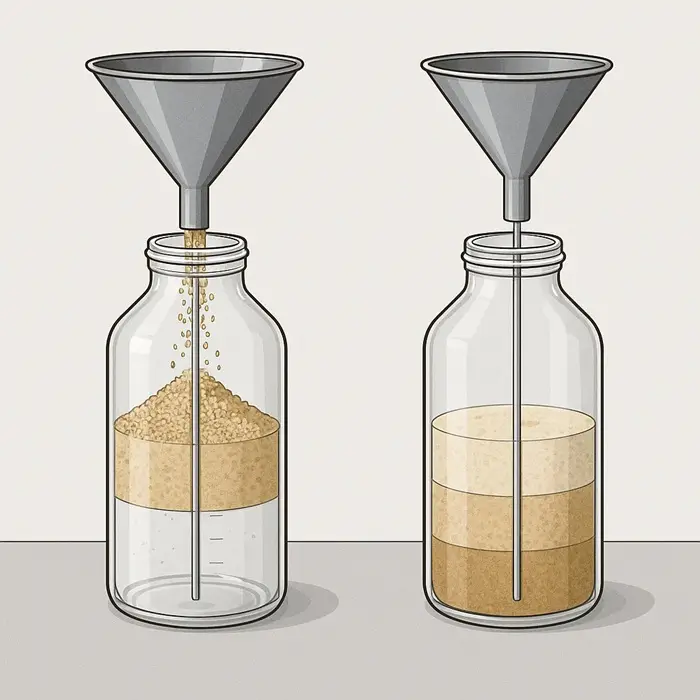
Two batches can share the same median particle size and moisture value, at least within the reported tolerances. One batch carries a long fines tail and fragile agglomerates that break easily during routine handling. Another batch holds robust granules with fewer fines and more stable structures that resist breakage under similar conditions. On the certificate, these batches look almost identical, although in a feeder, pneumatic line, or tablet press, they behave very differently.
The certificate also tells you nothing about handling history after production leaves the supplier. Drying conditions may have shifted slightly that week, or the product may have cooled more slowly inside a fuller silo. Transport might have added vibration, temperature swings, or humidity exposure during a long journey across regions. Each of these changes affects packing behavior, adhesion, electrostatic charge, and surface state, yet none of them appear in the standard document that arrives with the truck.
How quality systems really view supplier CoAs
Quality frameworks accept the use of supplier certificates of analysis, but they attach clear conditions that many teams quietly overlook. The receiving site remains responsible for the quality and fitness for use of incoming materials, regardless of who originally produced them. A certificate can replace some routine tests only after a formal supplier qualification process, and even then, performance must be monitored and reviewed using real data.
In practice, this means three things for a powder certificate of analysis, all of which are important during audits and customer visits. First, at least one identity check on each batch remains expected, even with an approved supplier and a long, stable history. Second, the receiving plant should verify that certificate data matches its own experience over a meaningful period, instead of assuming permanent stability. Third, any systematic drift between reported values and real performance should trigger investigation and corrective action, not excuses or informal workarounds on the line.
This mindset applies far beyond pharmaceuticals, active ingredients, or highly regulated environments with heavy documentation. Food, feed, battery materials, and speciality chemicals all face similar expectations from customers, brand owners, and regulators. A supplier certificate is one part of a risk-based control strategy, not a free pass to skip incoming verification, especially for powders that control process safety or key product properties.
ICH Q7 Good Manufacturing Practice guide for active pharmaceutical ingredients.
Designing a lean, powder focused intake panel
Incoming testing does not have to become a second full laboratory running complex methods on every truck. You can design a compact intake panel that still captures the main risks for powders, which influence flow, segregation, and stability. The goal is not to measure everything, because the goal is to detect meaningful changes before the material enters the main process and starts creating waste.
For many powders, a practical intake panel can include one identity or fingerprint test, for example, spectroscopy or a simple functional check. It includes a quick moisture or water activity measurement under defined conditions that reflect typical warehouse storage. A simple flow test that mimics your process, such as a small hopper or funnel, gives early insight into discharge behavior before powder reaches critical equipment. A rapid check for fines or oversize, using sieving or a fast optical method, exposes changes in particle size distribution shape that the certificate might hide. Finally, a structured visual inspection looks for caking, crusts, color shifts, or unusual agglomerates that hint at hidden history.
These tests are not exotic, and they do not need to be slow when procedures stay clear and well-trained. They can be standardized and executed within minutes, yet they reveal shifts that a routine certificate often hides completely. Changes in fines content, packing behavior, or cohesion appear quickly in these simple checks, long before they disrupt blending, conveying, or tableting performance. This only works when your intake samples are truly representative.
High-risk powders deserve deeper intake control that goes beyond this compact screening panel. Cohesive materials, hygroscopic blends, energetic powders, and combustible dusts belong to that critical group and justify extra effort. For these materials, it can be sensible to schedule periodic shear testing, wall friction measurements, or electrostatic characterisation using specialised equipment and external laboratories. Those tests do not have to run on every batch, because they can run at defined intervals or whenever a trend suggests trouble in either quality data or production performance.
When “same CoA” hides very different behavior
Consider a spray-dried excipient used in a premix that controls texture in the final product. To boost throughput before a seasonal peak, the supplier tightens drying conditions and pushes slightly harder on the production line to gain capacity. The certificate still reports assay, moisture, and particle size well within the agreed specification, so no one questions the batch at intake. However, particles become more brittle and internal structure changes, so during pneumatic conveying and silo discharge, more fines break off and accumulate.
Segregation then increases, and blend uniformity drifts towards the limits, even though nothing changed in the mixer recipe or batch size. Operators see more variability, while quality spends time proving that laboratory methods remain valid and precise. Without intake data, most discussions circle around equipment settings instead of material behavior, which delays corrective action and silently increases scrap.
Now imagine a conductive carbon black used in a slurry for electrodes or functional coatings in energy devices. A filter change in production leaves a slightly higher fraction of ultra-fine material in the product, which seems minor from the supplier’s perspective. The certificate values for structure and particle size remain within tolerance, yet in production, slurry viscosity rises and tap density falls noticeably. Coating control becomes more difficult, and energy density or conductivity drops for the finished part, even though the powder certificate of analysis appears normal and compliant.
In both cases, a plant that leans only on the certificate will likely blame the line instead of the material and lose valuable time. A plant that runs a modest intake panel will at least see that this batch is not like the last one before the powder reaches the most sensitive step. That early warning allows teams to pause, adjust, or divert the material before it undermines yields, schedules, or customer confidence.
Making CoAs more meaningful without making them longer
You cannot redesign every supplier quality system, but you can make certificates of analysis more relevant to your reality with a structured discussion. The first step is internal, so you start by mapping your actual failure modes using production records, trend charts, and complaint data. List the real issues from recent years, such as bridging in silos, excess dust, segregation in transfer, caking in storage, or coating defects on critical surfaces.
Then connect each failure mode to a measurable property that either you or the supplier can realistically track. Bridging often correlates with flow and consolidation behavior, while dust issues relate to fines content and electrostatics during filling and discharge. Segregation relates to density spread, particle size distribution, shape, and handling conditions throughout transport and internal transfer. Caking relates strongly to humidity exposure, contact mechanics, and storage time, especially in warm or coastal climates where conditions vary widely.
With that map in hand, review the certificate template with your supplier during the next technical meeting or audit visit. Discuss whether reported parameters link to the failure modes you care about and request values that say something about behavior, not purity alone. That might mean adding tapped density windows, reporting fines below a critical size fraction, or specifying flow index under defined humidity and temperature that resemble your warehouse. Each added field should connect directly to a real risk rather than general curiosity or tradition.
Finally, link certificate review to your intake data so both parties see the same picture over time. Stable intake results and stable performance can justify a reduced internal test frequency, which saves effort without increasing risk if monitoring continues. Drift in intake behavior, even with a stable certificate, should prompt discussion, investigation, and often updated agreements, especially when the powder is safety-critical or difficult to replace quickly.
From powder certificate of analysis to powder reality
A powder certificate of analysis is necessary for traceability, release decisions, and customer confidence in modern supply chains. It remains an important part of the chain, yet it still stays only paper until your process confirms the story printed on the page. Real confidence in powder behavior comes from seeing how the material performs under conditions that resemble your plant and your customers’ use.
When you treat the powder certificate of analysis as one pillar in a broader intake strategy, you reduce surprises downstream and protect capacity. You detect shifts while you still have options and time to respond, instead of reacting during an urgent shutdown with limited choices. You spend less time firefighting in mixers, dryers, and silos, and more time running the plant as intended with predictable, well-behaved powders that actually support your performance promises.