A catalyst can be defined as a substance that can be added to a process to increase the reaction rate without the catalyst getting consumed in the reaction process. Catalysts typically speed up the reaction process by reducing the activation energy or by changing the reaction mechanism. There are many types of catalyst and is in that sense the word catalyst is a “container term”, and they are generally defined in four groups, (1) Heterogeneous, (2) Homogeneous, (3) Heterogenized homogeneous catalysts, and (4) Biocatalysts such as enzymes and acid-based catalysts. Homogeneous and heterogeneous are referred to as surface catalysts. These surface catalysts are referred to as homogeneous or heterogeneous depending on whether they occupy the same phase as the chemical reaction mixture or not.
Generally, heterogeneous catalysts appear in the solid-state. Heterogeneous or surface catalysts are submerged in a liquid or gas chemical reaction mixture, the reactant adsorbs onto the binding site on the surface of the catalyst. Heterogeneous catalysis is very important to the industrial production of chemicals because of the enablement of selective product creation. The production of 90% of chemicals in terms of volume produced is made possible through solid catalysts. In this article, we will particularly look at these heterogeneous catalysts.
Heterogeneous catalysts and surface catalysis
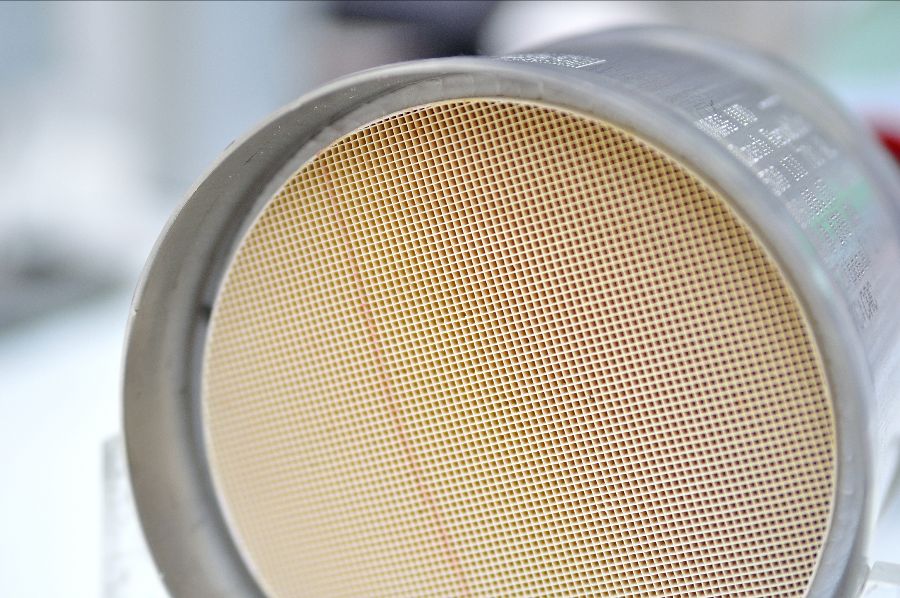
The common usage in the industry of heterogeneous and surface catalysis is the production process of plastics and polymers such as polyethylene. The catalysts used are called Ziegler-Natta catalysts and are used to make everything from plastic wrap materials to cups used in cup noodles for example. Another common example is the usage of a heterogeneous catalyst is the catalytic converter in your car. Catalytic converters include conversion metal catalysts fixed on a solid phase support, the solid-phase catalyst reacts with the gases from the car’s exhaust, increasing the reaction rate to form less toxic side-effects from pollutants in the exhaust stream such as carbon mono-oxide (CO).The catalytic converter is also a perfect example of a surface catalytic process, where the reactant molecules are adsorbed onto a solid surface before they interact with the catalyst to form the reactant product. The catalysis reaction rate increases depending on the surface area of the catalyst coming in contact with the reactants. That is why the catalytic converter is designed in such a way that the solids have a large surface area. The catalyst material is a very open honeycomb monolithic structure on which a high surface area carrier material is deposited which in turn can guarantee stabilization of highly dispersed catalytic species. In this way, a high surface area porous material can be coupled to a desired low-pressure drop.
Advantages and Disadvantages of Heterogeneous Catalysis
Heterogeneous catalysts have one distinct benefit regarding the production process. Heterogeneous catalysts are easily separated from a reaction mixture, for example through filtration, the catalysts can be effectively retrieved. Thus making it easy to streamline production processes.A clear disadvantage of heterogeneous catalysis is the saturation limit of the surface area. Once the surface of the catalyst is completely saturated with reactant molecules, the reaction process halts until the chemical reaction mixture leaves the solids porous surface in turn opening up spaces again for a new reactant molecule to be adsorbed or attached. Because of this, saturation is a rate-limiting factor in the industrial process in regards to the usage of heterogeneous catalysts.Despite this clear disadvantage, the benefits of heterogeneous catalysis generally outweigh the disadvantages, because the generated catalyzed reaction is still much more efficient in terms of reaction speed and selectivity than the uncatalyzed reaction.In order to understand the active site structures of a catalyst, it is crucial to examine the structure-activity relationships based on the physical properties of the catalyst, aspects such as porosity, pore size, surface area, and physical transport are all highly relevant in determining the effectiveness of the catalyst for its intended purpose or goal.
Delft Solids Solutions and the characterization of your catalysts
Catalyst characterization typically targets both the information on the catalyst support as well as the active metal phase. Physical gas adsorption is mostly used to cover the support properties and provides information on the specific surface area, the pore volume, and the pore size distribution of the catalyst carrier. This information can be crucial when it comes to the physical transport of molecules in and out of the catalyst and it will impact the catalyst effectiveness, but it can also relate to the formation of e.g. coke in the internal structure of the catalyst. The active phase present can be probed by chemical gas adsorption during which a reactive gas, often hydrogen or carbon mono-oxide, is used but this can also be a basic or acidic probe molecule.The former probe molecules are often used to obtain information on the active properties of the metal phase of a (supported) metal catalyst. It provides quantitative information on the active metal phase such as metal surface area, metal dispersion, and metal crystallite size, and enables to correlate the catalyst properties with its catalytic performance. In the case of acidic or basic catalysts counter probe molecules can be used to assess these types of sites. Please note that the size of the probe molecule can be very critical in a better understanding of the catalyst performance.If the probe molecule used in the characterization is e.g., much smaller than the reactant molecule used in the actual process, then the active surface area measured can be greatly overestimated due to improved access of the smaller probe molecule to the active sites compared to restricted access of the larger molecule used in the actual process. Besides active site accessibility, also physical transport in the catalyst particles is key. Clearly, such transport is greatly related to the porous structure of the catalyst and catalyst support.
Catalyst porosity by mercury porosimetry
Mercury porosimetry uses the non-wetting properties of mercury to gain information on the porous characteristics of catalysts, porosity, pore-volume, pore size distribution, and apparent density.The wide dynamic range over which pores can be investigated – pore sizes of 4 nanometers up to 800 micrometers – makes mercury porosimetry extremely useful to study powdered catalyst but also shaped entities such as extrudates, spheres, trilobes, etc. Delft Solids Solutions also provides the option to investigate the contact angle of mercury with the sample under investigation as this influences the pore size calculations and provides more accurate analysis results.
Transient uptake measurements for physical transport
As indicated, the physical transport of molecules in and out of the catalyst structure is of great importance. We can handle both gases and vapors (water and organic vapors) under a wide variety of conditions in terms of pressure and temperature to measure real-time the interaction with the catalyst material. Transient uptake or release curves provide the desired insight into how the porosity impacts the transport characteristics and ultimately the catalyst performance.
Want to know how we can help you?
We are always looking to interact with our peers, to optimize our Investigations for you. Our research is performed in close consultation with you and is executed under conditions that closely match the targeted application of the product or actual problem. Please feel free to reach out and we will gladly share our know-how and expertise.